High Temperature Alloy Report
Refractory Anchors and Hardware
JAYNE INDUSTRIES PERSPECTIVE:
As a supplier of alloy refractory anchors and hardware, Jayne Industries buys and processes high temp alloys to make finished parts. Jayne Industries represents the viewpoint of an enterprise which manufactures and services high temp alloy products, and receives real-world feedback about high temp alloy performance.
Jayne Industries does not design nor specify alloy grades, and has no particular allegiance to any supplier of alloys.
GOALS OF THIS PRESENTATION:
To promote a clearer understanding of the current state of commercially available high temp alloys which apply to refractory hardware.
To illustrate the characteristics of both traditional heat resisting stainless steel alloys, and the higher performance, true ‘high temp alloys’.
WHAT ARE HIGH TEMPERATURE ALLOYS?
Designed to maintain strength above room temperature.
Generally fulfill requirements for 500°F to 2200°F environments.
Alloying elements added to conventional stainless steels to improve properties at elevated temperatures.Originally driven by Aerospace developments.
DEFINITION:
Broadly speaking, high temperature alloys are metals designed to maintain strength above room temperature, and which generally operate between temperatures of 500°F and 2200°F.
HISTORY AND EVOLUTION:
In the early 1920’s, adding chrome to steel produced the first true high temperature materials, for resistance heating elements. Later, alloying with nickel was found to stabilize austenite, and promote protective chromia at lower chrome contents, giving the foundation for most of today’s high temperature alloys. Aerospace and gas turbine developments initially motivated the quest for improving the Fe-Cr-Ni alloys. This quest has since been accelerated by industry demands for continuous processing, reduced downtime, and the improved process efficiencies gained at increased temperatures. Today’s techniques used to improve high temp alloys include further alloying with molybdenum, cobalt, tungsten, aluminum, silicon, or rare earth elements (e.g. tantalum, cerium), and advanced melting techniques such as VIM-vacuum induced melting, VAR-vacuum arc remelting, and ESR-electroslag remelting.
MATERIAL PROPERTIES
• Thermal Expansion
• Thermal Conductivity
• Creep Strength
• Rupture Strength
• Thermal Fatigue Strength
• Thermal Shock Strength
• Erosion and Wear Resistance
• Corrosion Resistance
MATERIAL PROPERTIES AND HIGH TEMPERATURE ALLOYS
All metals expand when heated. Proper provision for expansion and contraction will reduce possibility of early mechanical failure. Thermal conductivity governs the rate at which localized heating is dissipated. Low thermal conductivity could produce distortion and burn-through in situations such as direct flame heating. Above 700°F, steel will flow continuously under applied load, rendering tensile and yield strengths inappropriate measures of material strength. Creep and stress-rupture are measures of elevated temperature strengths. Creep strength is commonly expressed as the stress to produce a 1% creep rate in 10,000 hours at a certain elevated temperature. The stress to produce rupture of a material over a certain amount of time, at a certain temperature, is called rupture strength. Thermal fatigue strength is the ability to survive cyclic temperature changes. Thermal shock resistance is the ability to survive rapid temperature change.
Erosion and wear resistance are important factors in abrasive or moisture-laden environments.
Corrosion and oxidation resistance are of primary importance, since high temperature materials must not deteriorate quickly at high temperatures.
TYPES OF CORROSION
• Pitting
• Galvanic
• Crevice
• General Corrosion
• Intergranular
• Stress-Corrosion Cracking
The ability of stainless steel to resist corrosion rests primarily in the ability of chrome to combine with oxygen to form a passive, protective film over the material. At high temperatures, this passive film may fail to protect the material by the following types or modes of corrosion:
• Pitting is a form of localized breakdown in the passive film, which is generally caused by the displacement of oxygen in the passive film by one of the halogen elements (e.g. chlorine, fluorine).
• Galvanic corrosion involves the transfer of metal between dissimilar materials, when electrically connected in an electrolytic solution.
• Crevice corrosion is another form of localized break in the passive film, caused by the buildup of contaminants (e.g. chlorides) in the absence of film-regenerating oxygen, which may occur in the closed sections between adjacent metal parts.
• General corrosion, intergranular corrosion, and stress-corrosion cracking are modes of corrosion explained in the following slides.
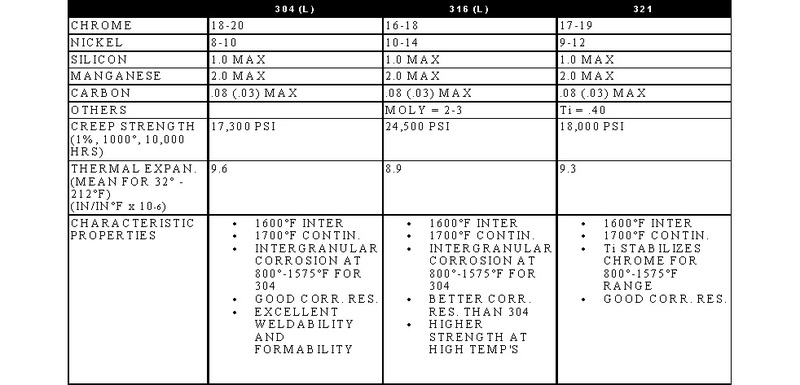
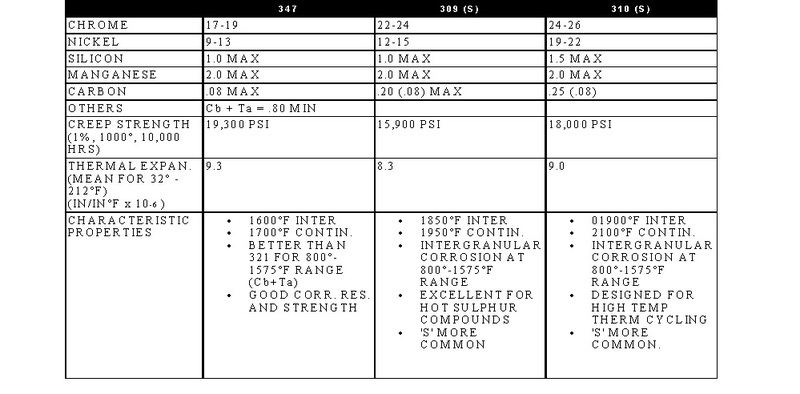
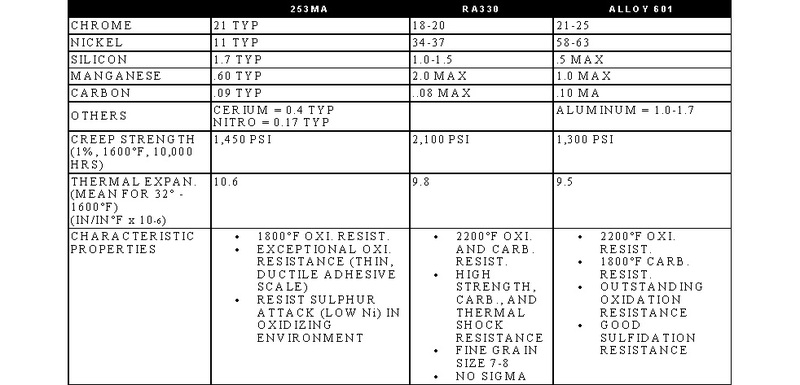
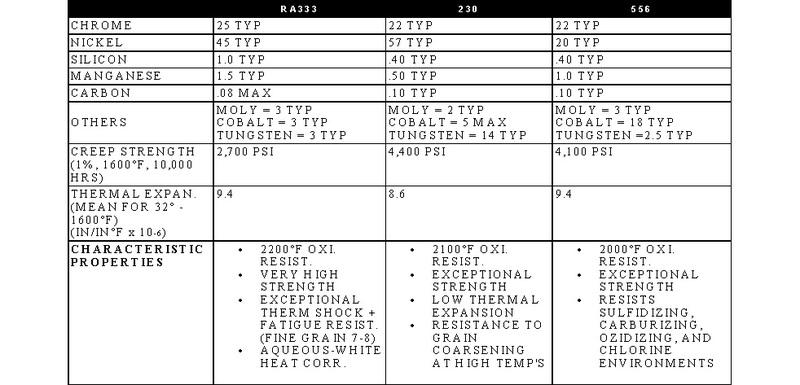
STAINLESS, HEAT-RESISTING, AND HIGH TEMPERATURE ALLOY
STAINLESS AND HEAT-RESISTING HIGH TEMPERATURE
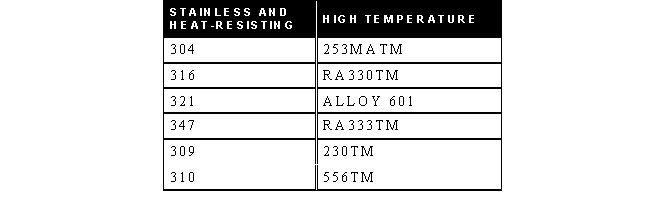
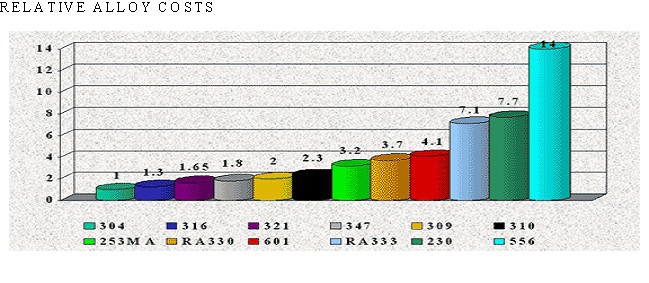
There are over 50 commercially available grades of heat-resisting and high temperature alloys. The alloys listed represent refractory hardware materials most commonly experienced by Jayne Industries, and those materials projected to have increasing usage in the near future. Of the alloys not discussed, many are suited to narrowly defined applications (particular corrodants), or are prohibitively expensive for furnace applications (e.g high cobalt or molybdenum alloys for severe corrosion or ultra-high temperatures). The alloys discussed are often available in many forms, including plate, sheet, bar, wire and tube.
- 253MA is a trademark of Avesta Sheffield.
- RA330 and RA333 are trademarks of Rolled Alloys.
- 230 and 556 are trademarks of Haynes International.